Why is Iron Important?
Iron is one of the most important nutritional requirements of the human body as it plays an important role in managing many vital biological processes. Low energy, poor concentration, and chronic fatigue are just a few of the many symptoms of iron deficiency.
Iron supplements are recommended by doctors for people who menstruate as well as during pregnancy to avoid the birth of low weight child and prenatal complications. Almost 10% of women between 12 and 49 have iron deficiency and athletes require more iron than the typical person. Iron supplements are used in various applications such as additional supplements, sports nutrition, and medicinal supplements, taken orally, or injected.

What’s Wrong with Existing Supplements?
Current iron supplements on the market are currently extremely hard on the body and cause many digestive side effects. This is because only a small percent of iron is absorbed in the intestines, causing the excretion of excess iron through the digestive tract where it stresses the body. The discomfort from taking iron supplements is enough to prevent many consumers from taking them as recommended by their health professionals. The iron supplement that is supposed to be easier to digest (ferric citrate) and the ones found in fortified food (ferric EDA), have been linked to a biomarker for cancer.
In October of 2020, Natural Products Insider, published an article discussing why the current products on the market are so insufficient and highlighting that the industry is overdue for a market disruptor.
Why is iron poorly absorbed?
A review paper by Li et al (2017) discusses the mechanisms for how iron is absorbed. The diagram to the right is from this paper. Basically, iron salts cannot dissolve easily in the liquid in the intestine and only enter cells passively. When iron is low enough to cause anemia, the intestinal cells will create a transporter protein to try to increase absorption, but this is often not enough. The unabsorbed iron ions from the salt pass through the digestive system and stress to the system, causing side effects. Therefore, more manufacturers are looking to bind iron to other compounds which can bind to existing transporter proteins to increase absorption and decrease side effects.
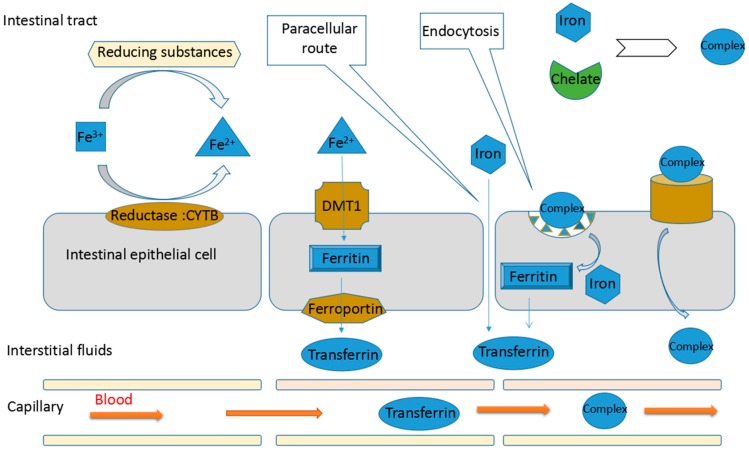


So how are consumers supposed to get iron if it is poorly absorbed by the body and not well tolerated by the digestive tract?
It is well understood by consumers that liquid iron is superior to tablet form, but that it is much more expensive. The other method of improving absorption is to bind (chelate) the iron to another compound that can cross the intestinal wall. The chelated form of iron “ferrous bisglycinate” seems to be acknowledged as having the least side effects, though it still causes some. The polysaccharide-iron complex is more difficult to find, and the heme iron polypeptide is becoming more popular. However, this would be unsuitable for vegetarians/vegans as it is derived from bovine hemoglobin.
When iron is bound to a polysaccharide, amino acid, or heme polypeptide, absorption is increased by one of three mechanisms: increased solubility, reduction of iron from one form to a more bioavailable form or promoting the passage of iron through intestinal cell membranes. Generally, the resulting increased absorption is due to a mix of all three.
Ecovatec’s Solution
While these other options do increase absorption and should decrease side effects, phosvitin peptides have a distinct advantage: the amino acid composition of PVP, specifically serine. PVP is unparalleled in metal binding and would be able to bind more iron and better increase its absorption. Even a small advantage in absorption allows for lower doses of iron which will drastically reduce digestive upset and stress.
Researchers (Feng and Mine, 2006) have demonstrated that phosvitin peptides (PVP) increase iron absorption by over 3x in a simulated intestine model (Caco-2 cell monolayer model). Ecovatec performed a similar experiment with our novel phosvitin peptide products, PVP and YPP to determine the appropriate dosage to optimize iron absorption. Preliminary results showed an 8x improvement in the intestinal cell iron levels when given PVP and iron sulfate compared to iron sulfate alone. The key to the research is optimizing the hydrolysis of the phosvitin into peptides, a process that Ecovatec has completed and is in the process of patenting. This novel hydrolysis produces very small peptides, critical to achieve improved iron absorption. Ecovatec is looking for partners interested in performing a clinical study, the results of which will allow for final formulation of an iron supplement with unprecedented absorption rates allowing for extremely low doses and no side effects. This new supplement recipe, featuring PVP, will completely disrupt the iron supplement market.
Sources are linked in the text above.


 A Canadian Company
A Canadian Company